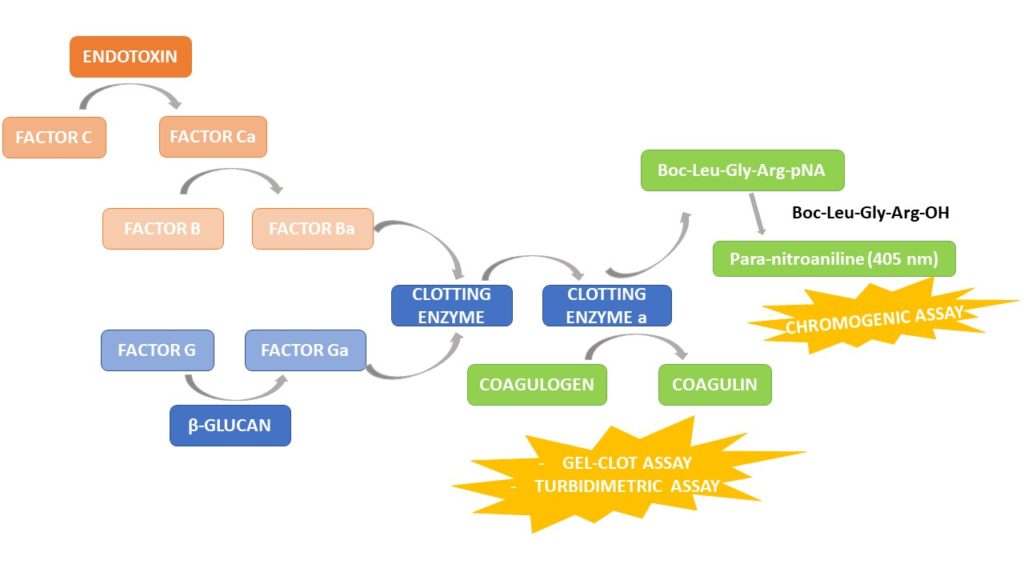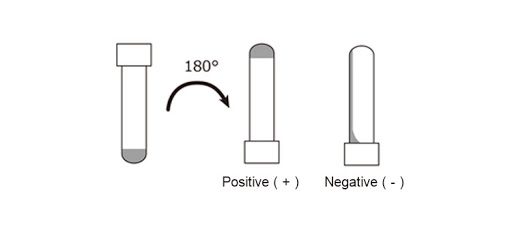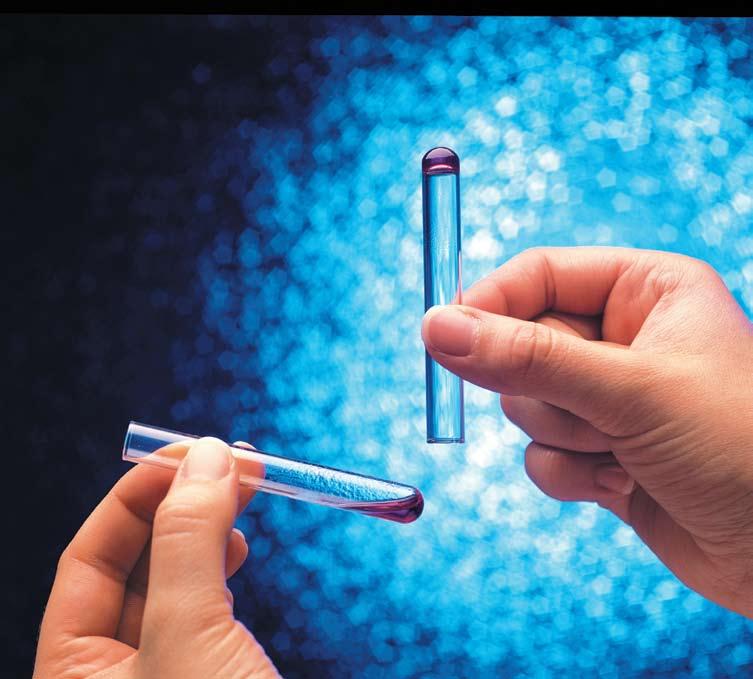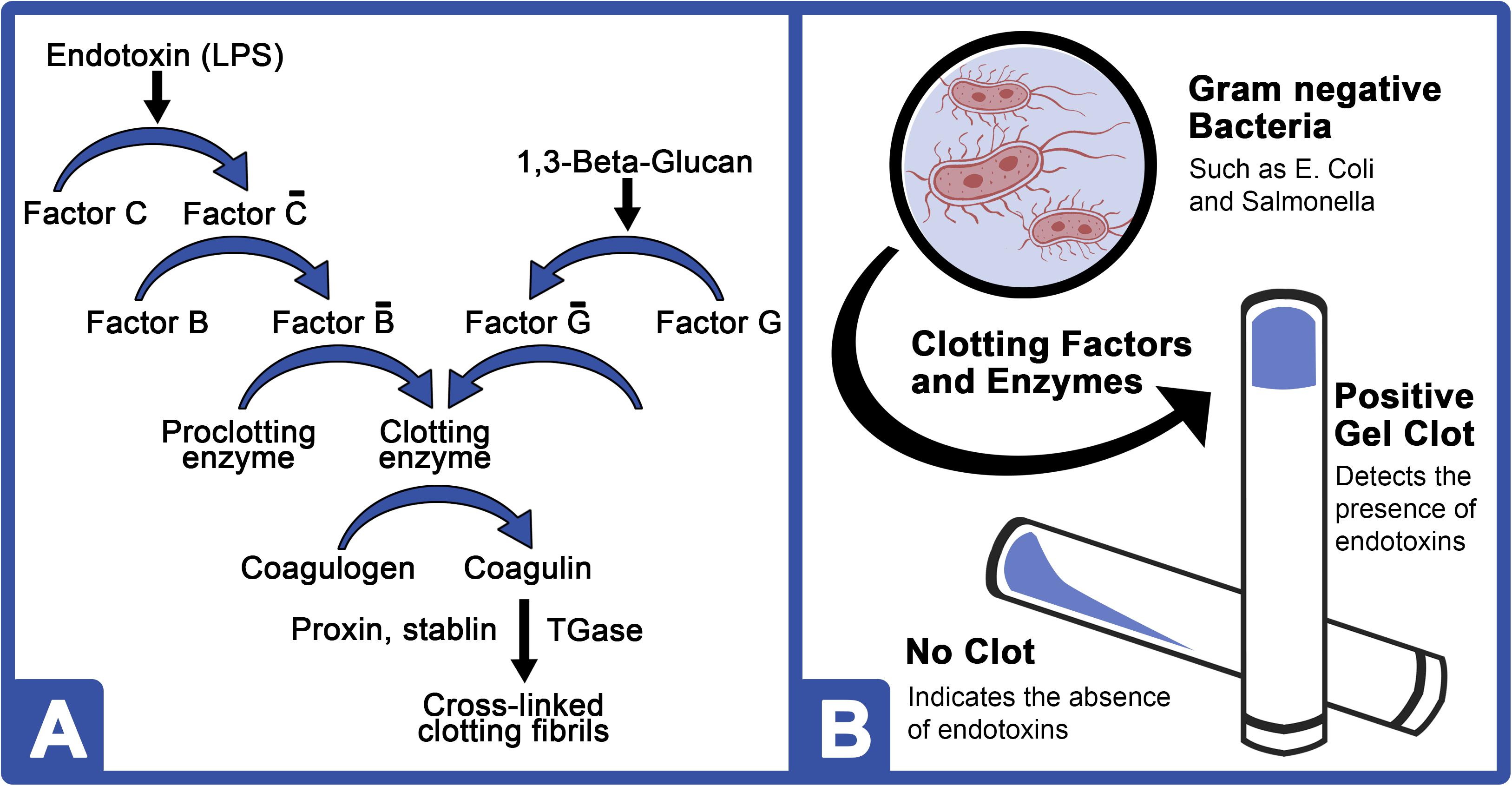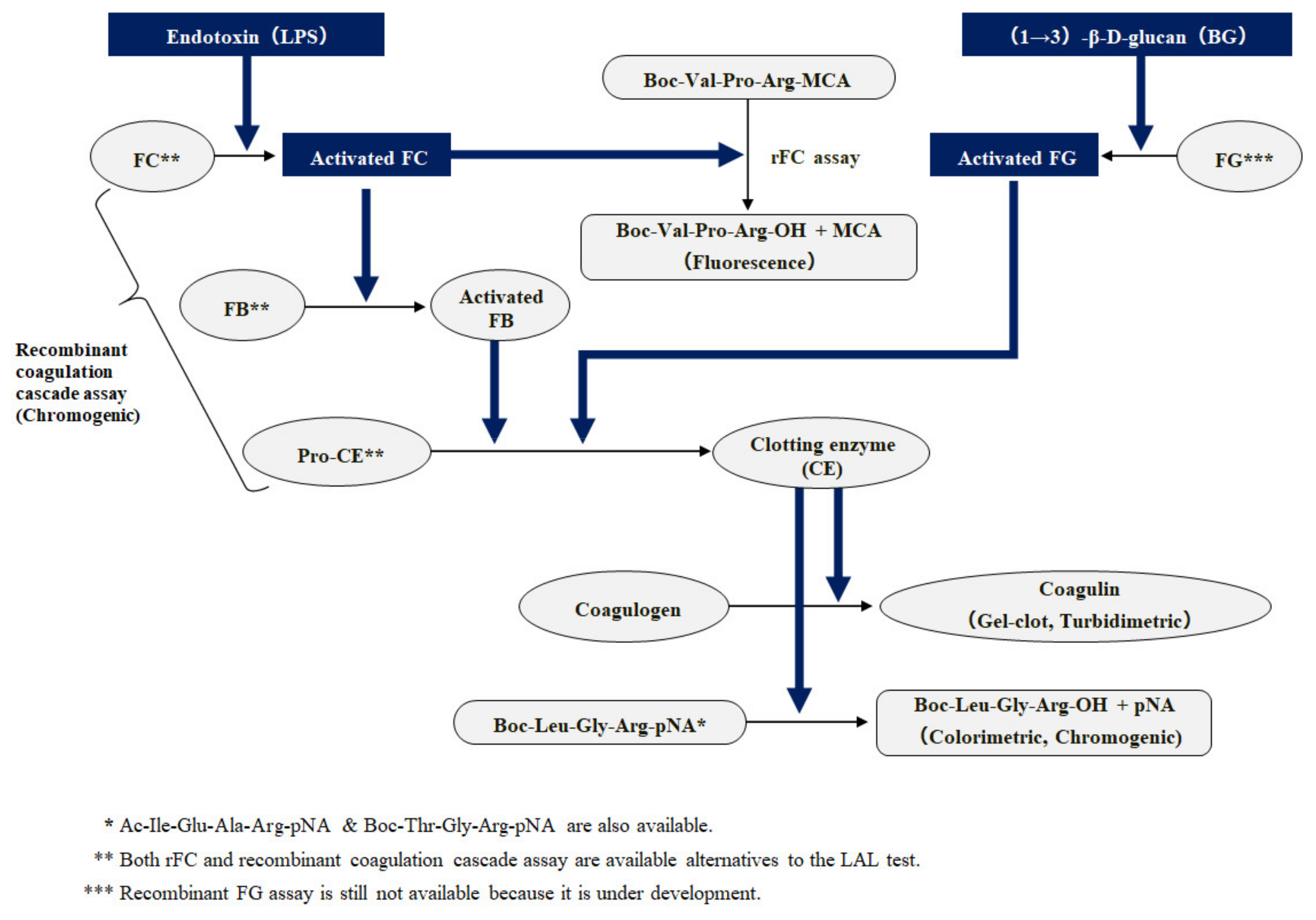
Biomedicines | Free Full-Text | Outstanding Contributions of LAL Technology to Pharmaceutical and Medical Science: Review of Methods, Progress, Challenges, and Future Perspectives in Early Detection and Management of Bacterial Infections and

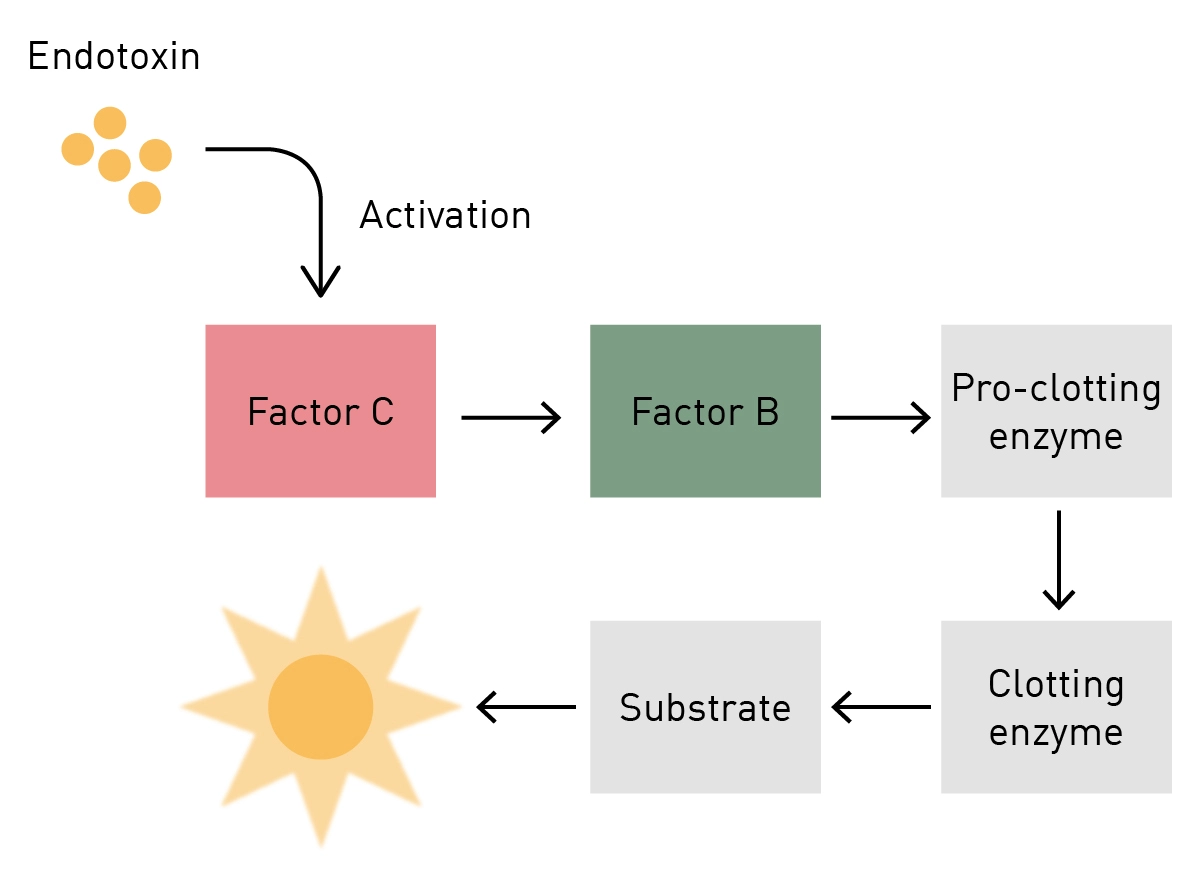
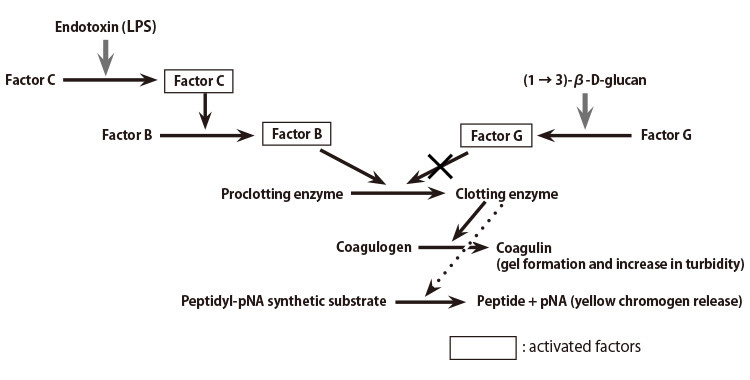
What Is Limulus Amebocyte Lysate (LAL) and Its Applicability in Endotoxin Quantification of Pharma Products | IntechOpen

LAL test validation protocol flow-chart. The test was performed three... | Download Scientific Diagram